
New class of recyclable polymer materials could one day help reduce single-use plastic waste.
Hundreds of millions of tons of single-use plastic ends up in landfills every year, and even the small percentage of plastic that gets recycled can’t last forever. But our group of materials scientists has developed a new method for creating and deconstructing polymers that could lead to more easily recycled plastics – ones that don’t require you to carefully sort out all your recycling on trash day.
In the century since their conception, people have come to understand the enormous impacts – beneficial as well as detrimental – plastics have on human lives and the environment. As a group of polymer scientists dedicated to inventing sustainable solutions for real-world problems, we set out to tackle this issue by rethinking the way polymers are designed and making plastics with recyclability built right in.
Why use plastics, anyway?
Everyday items including milk jugs, grocery bags, takeout containers and even ropes are made from a class of polymers called polyolefins. Polyolefins make up around half of the plastics produced and disposed of every year.
These polymers are used in plastics commonly labeled as HDPE, LLDPE or PP, or by their recycling codes #2, #4 and #5, respectively. These plastics are incredibly durable because the chemical bonds that make them up are extremely stable. But in a world set up for single-use consumption, this is no longer a design feature but rather a design flaw.
Imagine if half of the plastics used today were recyclable by twice as many processes as they are now. While that wouldn’t get the recycling rate to 100%, a jump from single digits – currently around 9% – to double digits would make a big dent in the plastics produced, the plastics accumulated in the environment and their capacity for recycling and reuse.
Recycling methods we already have
Even the plastics that make it to a recycling facility can’t be reused in exactly the same way they were used before – the recycling process degrades the material, so it loses utility and value. Instead of making a plastic cup that is downgraded each time it gets recycled, manufacturers could potentially make plastics once, collect them and reuse them on and on.
Conventional recycling requires careful sorting of all the collected materials, which can be hard with so many different plastics. Here in the U.S., collection happens mainly through single stream recycling – everything from metal cans, glass bottles, cardboard boxes and plastic cups end up in the same bin. Separating paper from metal doesn’t require complex technology, but sorting a polypropylene container from a polyethylene milk jug is hard to do without the occasional mistake.
When two different plastics are mixed together during recycling, their useful properties are hugely reduced – to the point of making them useless.
But say you can recycle one of these plastics by a different method, so it doesn’t end up contaminating the recycling stream. When we mixed samples of polypropylene with a polymer we made, we were still able to depolymerize – or break down the material – and regain our building blocks without chemically affecting the polypropylene. This indicated that a contaminated waste stream could still recover its value, and the material in it could go on to be recycled, either mechanically or chemically.
Plastics we need − but more recyclable
In a study published in October 2023, our team developed a series of polymers with only two simple building blocks – one soft polymer and one hard polymer – that mimicked polyolefins but could also be chemically recycled.
Connecting two different polymers together multiple times until they form a single, long molecule creates what’s called a multiblock polymer. Just by adjusting how much of each polymer type goes into the multiblock polymer, our team created a wide range of materials with properties that spanned across polyolefin types. But creating these multiblock polymers is easier said than done.
To link these hard and soft polymers, we adapted a technique that had previously been used only on very small molecules. This method is improved relative to traditional methods of making polymers in a step-by-step fashion, developed in the 1920s, where the reactive groups on the end of the molecules need to be exactly matched.
In our method, the reactive groups are now the same as each other, meaning we didn’t have to worry about pairing the ends of each building block to make polymers that can compete with the polyolefins we already use. Using the same strategy, applied in reverse by adding hydrogen, we could disconnect the polymers back into their building blocks and easily separate them to use again.
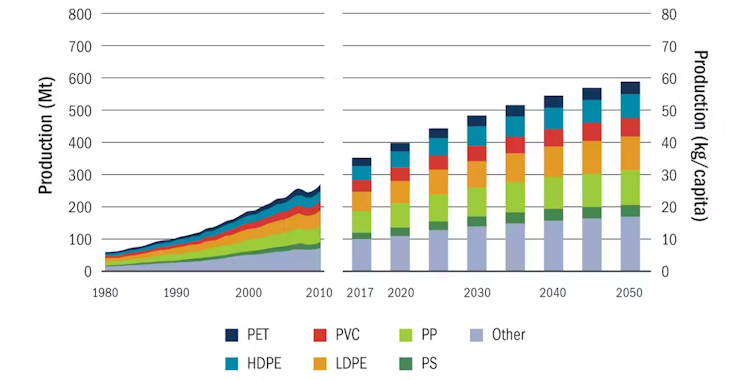
International Energy Agency
With an almost twofold increase in annual plastic use projected through 2050, the complexity and quantity of plastic recycling will only increase. It’s an important consideration when designing new materials and products.
Using just two building blocks to make plastics that have a huge variety of properties can go a long way toward reducing and streamlining the number of different plastics used to make the products we need. Instead of needing one plastic to make something pliable, another for something stiff, and a third, fourth and fifth for properties in between, we could control the behavior of plastics by just changing how much of each building block is there.
Although we’re still in the process of answering some big questions about these polymers, we believe this work is a step in the right direction toward more sustainable plastics.
We were able to create materials that mimic the properties of plastics the world relies on, and our sights are now set on creating plastic compositions that you couldn’t with existing methods.![]()
Katherine Harry, Colorado State University and Emma Rettner, Colorado State University
Katherine Harry, PhD Student in Chemistry, Colorado State University and Emma Rettner, PhD Candidate in Materials Science and Engineering, Colorado State University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Every product is selected by editors. Things you buy through our links may earn us a commission.

